News
August 30, 2025
Edwards Lifesciences celebrates new ESC guidelines
Edwards Lifesciences Corp. execs no doubt danced a little jig on the release of the latest European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines for valvular heart disease. The update established a simplified pathway for severe aortic stenosis (AS) that eliminated the previous divide between symptomatic and asymptomatic severe AS.
**Edwards Lifesciences Sees Boost as New Heart Valve Guidelines Simplify Treatment**
Medical technology company Edwards Lifesciences is celebrating the recent release of updated guidelines from the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) regarding the treatment of valvular heart disease. The new guidelines, specifically addressing severe aortic stenosis (AS), are being hailed as a significant step forward in streamlining patient care and potentially broadening access to life-saving treatments.
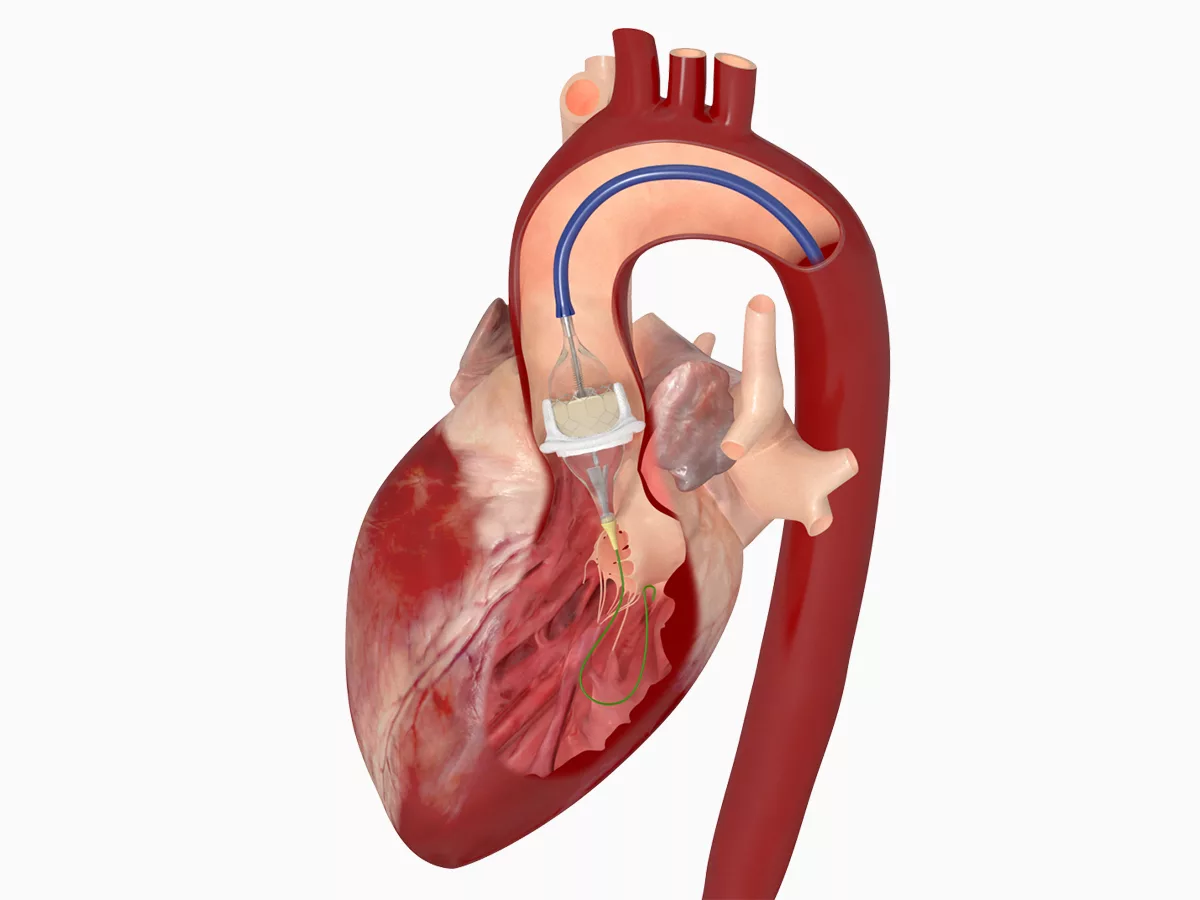
Aortic stenosis is a serious condition where the aortic valve, the heart’s main gate for blood flow to the body, narrows. This narrowing restricts blood flow, forcing the heart to work harder and potentially leading to heart failure and other complications.
The key change in the updated guidelines centers around the classification and treatment pathway for severe AS. Previously, a distinction was made between patients experiencing symptoms related to their severe AS and those who were asymptomatic, meaning they showed no obvious signs of the disease. This division often dictated treatment options and timing.
The new guidelines eliminate this strict symptomatic versus asymptomatic divide, establishing a more simplified pathway for managing patients with severe AS. This simplification means doctors can now consider a broader range of factors when deciding on the best course of treatment for each individual, potentially leading to earlier intervention and improved outcomes.
Edwards Lifesciences, a leading manufacturer of heart valve therapies, stands to benefit from this shift. The simplified pathway could lead to increased adoption of transcatheter aortic valve replacement (TAVR), a minimally invasive procedure to replace the damaged aortic valve, a key area of focus for the company. While the guidelines do not explicitly endorse TAVR, the streamlined approach to patient management could encourage its use in a wider range of appropriate patients.
The company is undoubtedly pleased with the updated guidelines, viewing them as a positive development for both patients and the advancement of heart valve therapy. Experts anticipate that the new ESC/EACTS guidelines will provide clinicians with clearer direction and ultimately lead to better care for individuals suffering from severe aortic stenosis across Europe.
Medical technology company Edwards Lifesciences is celebrating the recent release of updated guidelines from the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) regarding the treatment of valvular heart disease. The new guidelines, specifically addressing severe aortic stenosis (AS), are being hailed as a significant step forward in streamlining patient care and potentially broadening access to life-saving treatments.
Aortic stenosis is a serious condition where the aortic valve, the heart’s main gate for blood flow to the body, narrows. This narrowing restricts blood flow, forcing the heart to work harder and potentially leading to heart failure and other complications.
The key change in the updated guidelines centers around the classification and treatment pathway for severe AS. Previously, a distinction was made between patients experiencing symptoms related to their severe AS and those who were asymptomatic, meaning they showed no obvious signs of the disease. This division often dictated treatment options and timing.
The new guidelines eliminate this strict symptomatic versus asymptomatic divide, establishing a more simplified pathway for managing patients with severe AS. This simplification means doctors can now consider a broader range of factors when deciding on the best course of treatment for each individual, potentially leading to earlier intervention and improved outcomes.
Edwards Lifesciences, a leading manufacturer of heart valve therapies, stands to benefit from this shift. The simplified pathway could lead to increased adoption of transcatheter aortic valve replacement (TAVR), a minimally invasive procedure to replace the damaged aortic valve, a key area of focus for the company. While the guidelines do not explicitly endorse TAVR, the streamlined approach to patient management could encourage its use in a wider range of appropriate patients.
The company is undoubtedly pleased with the updated guidelines, viewing them as a positive development for both patients and the advancement of heart valve therapy. Experts anticipate that the new ESC/EACTS guidelines will provide clinicians with clearer direction and ultimately lead to better care for individuals suffering from severe aortic stenosis across Europe.
Category:
Technology